Abstract
Introduction: Venous thromboembolism (VTE), defined as deep venous thrombosis (DVT) and pulmonary embolism (PE), is a cause of significant morbidity and mortality worldwide, with an overall incidence of about 10,000,000 cases per year. The majority of VTEs are believed to be attributable to genetic factors. Yet, the five established heritable thrombophilias of factor V Leiden (FVL), prothrombin (PT) gene mutation, antithrombin (AT) deficiency, protein C (PC) deficiency, and protein S (PS) deficiency comprise only a small portion of VTEs, suggesting that further genetic factors contributing to VTE risk are unrecognized. In our previously published study, we performed whole exome sequencing (WES) in 64 patients with VTE and developed a 55-gene extended thrombophilia panel which identified 40 pathogenic or likely pathogenic variants or variants of uncertain significance (VUS) involving 22 different genes. Here, we present updated data from an expanded patient cohort.
Methods: Blood was obtained from 101 patients with VTE. Genomic DNA was extracted and WES performed; mean coverage of the exome was 100x with 98% of the exome covered >/= 20 times. Variants were filtered for an allele frequency of 7% in the GnomAD database. A targeted analysis of the 55 genes in the extended thrombophilia panel was then performed. Variants in these genes were classified according to ACGM criteria as pathogenic, likely pathogenic, VUS, benign or likely benign. The number of patients with pathogenic or likely pathogenic variants and VUS in VTE patients was compared to a control population of 237 patients who had WES performed for reason other than VTE. The results of WES were also compared to those of traditional laboratory-based thrombophilia testing. Further, 17 VUS underwent in silico protein modeling to evaluate structural modifications.
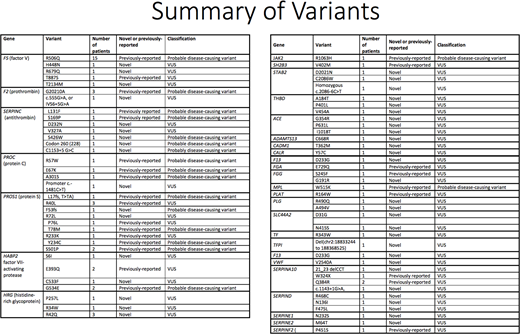
Results: Of the 101-patient study population, 46 were men and 55 women; 62% were Caucasian, 22% African American and 5% Hispanic; 15% had unprovoked VTE, 71% had provoked, 12% had both; and 55% had first degree family members with VTE. WES and extended thrombophilia testing identified a pathogenic or likely pathogenic variant or VUS in 69/101 (68%) VTE patients compared to 6/237 (2.5%) controls, a statistically significant difference (p-value <0.001). 86 patients underwent traditional laboratory-based thrombophilia testing; of these, 35 patients had a positive laboratory abnormality, with 31/35 (89%) also demonstrating a variant on WES. Among these 35 patients, in instances when laboratory-based thrombophilia testing did not correlate with WES, the abnormal lab was usually a borderline low PC or PS level that was not interpretable due to the clinical scenario (e.g. warfarin use). 51 patients had negative laboratory-based thrombophilia testing, of whom 30/50 (58%) had a variant on WES.
A total of 72 genetic variants (26 previously published and 46 novel) were identified in 30 genes in the extended thrombophilia panel. 18 variants were categorized as pathogenic or likely pathogenic and 54 as VUS. 32 patients had pathogenic or likely pathogenic variants in one or more of the major thrombophilia genes, with FVL being the most common, and AT and PS deficiency being more common than PT gene mutation (FVL, n=15; PT gene mutation, n=3; AT deficiency, n=5; PC deficiency, n=3; PS deficiency, n=6). Of pathogenic variants in major thrombophilia genes, 15 were previously reported in literature, while 13 were novel. Of the 52 VUS, 17 were subjected to in silico analysis, including protein modeling, which suggested impaired protein folding in 14 variants.
Conclusions: WES and extended thrombophilia testing reveal a high frequency of pathogenic or likely pathogenic variants or VUS in VTE patients compared to controls. These variants demonstrate good concordance with traditional laboratory-based thrombophilia testing. Several novel pathogenic or likely pathogenic variants were identified. In silico studies and protein modeling suggest that many VUS identified by this approach are likely to be deleterious to protein function. The results may underlie a strong genetic component to VTE. Next steps include further characterization of VUS using protein modeling, biochemical analysis, and epidemiological and familial studies to understand potential roles these variants may play in thrombosis.
Camire:Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Spark Therapeutics: Membership on an entity's Board of Directors or advisory committees; Bayer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal